Prostate Cancer - Advanced

Overview
This article was developed to provide general information about the treatments available at UCSF for patients with advanced prostate cancer. Our Prostate Cancer Center has a strong commitment to delivering state-of-the-art care, improving existing treatments, and developing new therapies for all stages of prostate cancer.
Introduction
Prostate cancer is considered advanced when it requires additional treatment beyond radiation or surgery. "Advanced prostate cancer," however, covers a range of disease. Some advanced cancer patients have metastases, meaning the disease has spread to distant sites, such as lymph nodes, organs and bones. Others have no metastases seen on imaging tests but their PSA rises after initial treatment, a condition known as rising-PSA or PSA-only prostate cancer. While most of the treatments described below are for patients with metastases, they may also be offered to patients with rising-PSA cancer.
Your choice of treatment is influenced by several factors, including your current medical condition, treatments you've already received, location and extent of your cancer, and presence or absence of symptoms. For patients with PSA-only cancer, the prostate-specific antigen (PSA) doubling time is also considered.
Another factor is how your cancer responds to hormone therapy, which is described in the next section. Generally, as long as your cancer responds to hormone therapy, other treatments will not be required. However, many cancers will eventually progress despite hormone therapy, and when that occurs the more advanced therapies described later in this article will be indicated.
Awards & recognition
-

Among the top hospitals in the nation
-

Best in Northern California for cancer care (tie)
-

Best in Northern California for urology
-
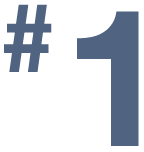
in NIH funding for urology research
-

Rated high-performing hospital for prostate cancer surgery
Hormone therapy
Hormone therapy is frequently the first treatment offered to patients with metastatic prostate cancer. It is also an option for some patients who choose not to have surgery or radiation for cancer confined to the prostate and for some patients who have a rising PSA after surgery or radiation (or both treatments).
The hormone testosterone promotes prostate cancer growth. Reducing testosterone levels in the body with hormone therapy can weaken and may kill prostate cancer cells. Testosterone is primarily produced by the testicles, though the adrenal glands also produce a small amount. One way to reduce testosterone production is through surgical removal of the testicles, a procedure known as orchiectomy. It is rarely done today but remains an option.
The more common method is androgen deprivation therapy (ADT), which uses medications to stop production of testosterone and other androgens from the testes. These include Lupron and Eligard (brand names for leuprolide acetate), Zoladex (goselerin) and Firmagon (degarelix) – all administered by injection. In 2020, the Food and Drug Administration (FDA) approved the first oral testosterone-lowering medication, Orgovyx (relugolix), which works in a similar manner to Firmagon. These medications are as effective as orchiectomy in reducing testosterone levels and treating prostate cancer, and they have the advantage that the testosterone level can recover if the treatment is paused or discontinued. Metastatic patients can continue on ADT for years or even decades, but it is often combined with other therapies early in treatment, as discussed below.
A person whose disease is responding to ADT is considered to have ADT-sensitive prostate cancer. A person on ADT whose disease is growing is considered to have ADT-resistant cancer. In other literature, for historical reasons, ADT-resistant disease is sometimes referred to as "castration-resistant" disease.
Patients who begin ADT with Lupron, Eligard or Zoladex (or Trelstar, Vantas or Viansa, which are similar drugs) will sometimes be given another testosterone-lowering drug, Casodex (bicalutamide), for two weeks at the start of therapy to counter a phenomenon known as testosterone flare that can briefly cause pain in patients who have metastases in the spine or other bones at-risk. Casodex is not needed with Firmagon or Orgovyx.
A few different strategies are used for administering ADT. While many patients stay on ADT continuously, some have the option of an intermittent regimen that allows them to cycle on and off the testosterone-lowering drugs. With this approach, you take the drugs until your PSA falls to its lowest point and then continue treatment for another nine to 12 months. The drugs are then stopped, and your PSA is carefully monitored, typically with testing every one to three months. When your PSA rises to a level predetermined by you and your oncologist, you resume ADT for another nine to 12 months, at which point you can take another drug holiday. The PSA is again allowed to rise to a predetermined level, and the cycle continues. The main benefit of this approach is getting a break from hormone therapy's side effects.
We most often use intermittent ADT in patients without metastasis. In patients with metastasis, intermittent ADT therapy isn't as effective as continuous therapy. Your doctor will discuss with you whether you are an appropriate candidate for the intermittent approach.
Side effects of hormone therapy
Whether through surgery or medication, hormone therapy can cause side effects that include hot flashes, low sexual desire, erectile dysfunction, fatigue, mood changes, muscle loss, weight gain and anemia.
Patients on long-term hormone therapy also have a risk of osteoporosis (weakening of the bones). To maintain bone health, ask your doctor about taking a calcium or vitamin D3 supplement. Your blood levels should be evaluated, as low vitamin D levels are common, and some individuals require a higher dose of this supplement. You should also participate in weight-bearing exercise regularly, as it helps to maintain bone health and muscle tone as well as reduce fatigue.
In addition, ADT's effects on metabolism may increase your risk of diabetes and heart disease. We strongly recommend both aerobic and resistance exercise to maintain metabolic health, cardiovascular health, bone strength and quality of life. While treatment for advanced prostate cancer can disrupt your daily routine, it's important not to abandon the healthy diet and exercise practices that are key to wellness and survival.
ADT's impact on sex life is as important as the other side effects, and we hope to provide an open, supportive environment for you to discuss your condition and concerns. UCSF offers a program for treating erectile dysfunction.
Combination therapy
In the past, the first treatment for metastatic prostate cancer was ADT alone. Other treatments would be added when the disease became ADT-resistant – that is, when ADT no longer controlled the cancer. Although there are still cases where it's appropriate to start treatment with ADT alone, several studies, beginning in 2017, showed a survival advantage to combining ADT with a more advanced treatment early on, before the disease became ADT-resistant. You and your doctor should discuss the combination treatments that are best for you, looking at relative benefits and side effects.
Unfortunately, metastatic disease typically mutates over a number of years and becomes resistant to such combination treatments. Other treatment options (described in later sections) can be applied when this occurs and this is an active area of research.
The three treatments commonly combined with ADT in metastatic therapy are:
1. Androgen signaling inhibitors are a new class of antiandrogens that were introduced in 2011 and originally approved to treat ADT-resistant disease, though can now be used to treat ADT-sensitive disease as well.
- Zytiga (abiraterone acetate) shuts down androgen production in the adrenal gland and inhibits tumors from manufacturing their own testosterone. It's taken orally once a day in conjunction with prednisone. This combination has been shown to prolong survival in patients who have previously received chemotherapy, and it's also of great benefit to those just beginning treatment for metastatic or those whose prostate cancer has spread only to near-by lymph nodes. The FDA has approved it for both of these settings. Most patients tolerate Zytiga well. Side effects can include increased blood pressure, fluid retention, elevation in liver enzymes, and electrolyte abnormalities.
- Xtandi (enzalutamide) blocks testosterone's stimulating effects on prostate cancer cells. In patients who have previously received chemotherapy, Xtandi has been shown to improve survival, PSA response, and tumor response. It is approved by the FDA for patients with metastatic prostate cancer, whether ADT-sensitive or not. It's also approved for patients who experience a rising PSA while on ADT, even if no metastasis has been detected. The drug is taken daily by mouth and is well tolerated. There are rare reports of seizures in patients treated with Xtandi. You should discuss any history of seizures with your health care provider.
- Erleada (apalutamide) is another antiandrogen pill that blocks testosterone's effects. It significantly improves survival in patients with metastatic ADT-sensitive disease and in patients with rising-PSA cancer that's resistant to ADT. The FDA has approved Erleada for these patients. Side effects include high blood pressure, elevated glucose levels, elevated cholesterol levels and (particularly in the elderly) increased falls.
- Nubeqa (darolutamide) is an antiandrogen that has fewer neurological side effects than other antiandrogens because it doesn't cross the blood-brain barrier. It's currently approved by the FDA for ADT-resistant prostate cancer where the only evidence of disease is a rising PSA. The drug is taken by mouth twice daily.
- Casodex (bicalutamide) and a few other medications are less commonly used.
2. Chemotherapy. Usually administered intravenously in our infusion clinic, chemotherapy drugs directly kill prostate cancer cells. Many hospitals offer newly diagnosed metastatic patients a choice between ADT plus chemotherapy and ADT plus a second-generation antiandrogen.
- Taxotere (docetaxel) is a chemotherapy medication given intravenously every three weeks in conjunction with prednisone (taken orally twice a day). The standard duration of treatment is six cycles, for a total of 18 weeks. The combination of Taxotere and prednisone has been shown to prolong survival in patients with metastatic ADT-resistant disease as well as in those who have newly diagnosed metastatic prostate cancer and are initiating ADT. In patients with ADT-resistant cancer, approximately 50 to 60% of patients treated with Taxotere will have a significant decrease in PSA, and 20 to 40% will have shrinkage of measurable tumors. Side effects may include fatigue, fluid retention, nausea and neuropathy (nerve damage). Usually occurring after many doses, the neuropathy is typically experienced as numbness or tingling in fingers and toes.
- Jevtana (cabazitaxel) is a chemotherapy option for patients with or without prior treatment with Taxotere. Like Taxotere, Jevtana is given every three weeks in conjunction with twice-daily prednisone. The FDA has approved the use of Jevtana and prednisone only for patients that have already received Taxotere. Major side effects may include diarrhea, low blood counts, and immune system impairment that increases the risk of serious infections. Due to that infection risk, Jevtana is often given with a medication called Neulasta (pegfilgrastim) that can boost the immune system. There are other chemotherapeutic options that can be used after – or in combination with – Taxotere and Jevtana. While these drugs haven't been shown to improve survival, they help some patients control symptoms and reduce or stabilize the tumor. Your health care provider will explain these options if they are suitable for you.
- Paraplatin (carboplatin) is a chemotherapy drug that may be added to either Taxotere or Jevtana in certain cases.
3. Radiation therapy. Until recently, patients who already had metastases at diagnosis weren't offered radiation therapy or surgical removal of the prostate (prostatectomy). However, a recent randomized controlled trial has shown that for patients with a low metastatic burden, radiation treatment to the prostate can result in a significant survival benefit. For patients with a higher metastatic burden, no such benefit has been observed. In addition, advances in imaging and precision radiation techniques have made it possible to know the extent of metastases and treat them with targeted radiation or surgery if the number of detected metastases is fewer than five. (This condition of limited spread is called oligometastasis). Studies have also shown radiation therapy offers a survival benefit when metastasis is confined to the pelvic lymph nodes (when the condition is called regionally advanced). Studies that treat more distant metastases are ongoing but haven't yet yielded conclusive results.
UCSF webinar
Initial treatments for metastatic prostate cancer
Dr. Rahul Aggarwal discusses state-of-the-art treatment for metastatic disease.
Advanced therapies
If cancer progresses despite the combination therapies described above, three additional types of therapy can be used:
1. Immunotherapy. These agents stimulate your body's own immune system to fight your cancer. While usually well-tolerated, they aren't effective for everyone.
- Provenge (sipuleucel-T) is custom-made from your own immune cells. First, for two to three hours, the patient's blood is run through a machine in an outpatient infusion center to extract certain immune cells. These immune cells are then mixed with a protein commonly found on prostate cancer cells, which sensitizes the immune cells to the cancer. Two days after the immune cells were harvested, the mixture is returned to the patient in an hour-long infusion. The whole process is repeated two more times over the course of a month. This cancer vaccine tells your immune system that prostate cancer cells should be attacked as if they were foreign invaders. In clinical trials, patients with hormone-refractory prostate cancer who received Provenge lived an average of four months longer than those who did not. Some patients were alive three years later. Interestingly, patients who lived longer with Provenge did not have PSA reductions or tumor shrinkage. Side effects of Provenge are mild; they include flulike symptoms that resolve within a few days and (rarely) allergic reactions at the time of infusion.
- Keytruda (pembrolizumab) has shown benefit in several advanced cancers that have abnormal DNA repair mechanisms, which is when genes regulating DNA (called mismatch repair genes) don't work correctly. The mismatch repair genes work like the body's spellcheckers and correct errors in DNA. When these genes stop functioning normally, DNA errors aren't repaired, resulting in the cancer cells becoming unstable and therefore more likely to respond to Keytruda. The FDA approved Keytruda for treating advanced cancers (including prostate) that have deficient mismatch repair and that continue to progress when all other treatment options have been exhausted. Keytruda is especially effective in patients whose tumor biopsy shows an indication labeled "MSI-H" (for microsatellite instability – high). Keytruda is also being applied with other therapies in clinical trials because it can help the body's own immune cells penetrate the cancer's defenses.
2. Radioisotope (radioactive isotope) therapy. Radioactive agents can be used to target and kill cancer cells.
- Xofigo (radium-223) is a radioactive drug that can be taken up in the bones in place of calcium (radium is chemically similar to calcium). Once it's incorporated into the bone, radium-223 emits radiation that can kill nearby metastatic cells growing in bones. A study demonstrated that six treatments with this isotope can decrease bone-related complications of the cancer as well as improve survival. It is typically used in patients who either have already received chemotherapy or aren't healthy enough for chemotherapy. Injected intravenously, Xofigo targets areas of bone that are being damaged by cancer. For most patients, side effects are mild, such as mild diarrhea. However, it's important to note that the patient's bone marrow health needs to be monitored with regular blood tests.
- Pluvicto (Lutetium-177 targeting PSMA) is a radioisotope therapy that targets a protein called PSMA (prostate-specific membrane antigen), which grows on the surface of prostate cancer cells. Over the past decade, new molecules have been developed that can attach radioactive isotopes to prostate cancer cells by targeting their PSMA proteins. When the isotope gallium-68 is used this way, it permits a PSMA PET scan to detect the cancer cells with much more accuracy and sensitivity than do other imaging technologies. When the radioactive isotope lutetium-177 is attached to the cells' PSMA, it damages and eventually kills the cancer cells. Some prostate cancer cells (approximately 5 to 10%) do not express PSMA on their surface, will not show on a PSMA PET scan, and can survive treatment with PSMA-directed lutetium-177.
3. DNA repair targeting. When a cell's DNA is damaged, a mechanism within the cell tries to repair the DNA. About 25% of patients with ADT-resistant disease have gene mutations – either inherited or acquired – that disable this repair mechanism.
- The most commonly mutated genes include BRCA1, BRCA2, ATM and CHEK2. Fortunately, patients with these mutations can be treated with a chemotherapy drug called carboplatin or with a class of drugs called PARP inhibitors. Patients with BRCA2 mutations appear to benefit the most from PARP inhibitors.
- Lynparza (olaparib) and Rubraca (rucaparib) have been approved for treatment of ADT-resistant prostate cancer associated with one of the common gene mutations. Your health care provider will discuss these options if they are suitable for you.
Investigational therapies
1. UCSF has an extensive clinical trials program available to virtually all patients who volunteer and qualify. Some studies are investigating experimental therapies not approved by the FDA; others are using FDA-approved therapies in new ways or in combination with experimental agents. There are many ongoing clinical trials at UCSF. In general, to be eligible for most clinical trials, your prostate cancer must be progressing. If you're responding to a particular treatment, participation in a clinical trial may not be possible at the present time but may be appropriate in the future. Your health care provider can talk to you about any in which you may be eligible to participate; you can find more information about any UCSF prostate cancer study at UCSF Prostate Cancer Clinical Trials — San Francisco Bay Area.
2. UCSF has a large program focused on obtaining biopsies from sites of metastatic disease, such as the bone and liver, from patients with metastatic prostate cancer. This important research can help us more accurately describe the types and subtypes of prostate cancer that evolve over months and years of treatment, which may eventually enable us to tailor therapies for individual patients. Your health care provider may talk to you about participating in our biopsy program.
3. UCSF has a number of experimental programs to analyze tumor cells circulating in the blood, many as part of other experimental protocols.
4. UCSF has a large phase I Developmental Therapeutics Program led by an experienced team of experts in many medical subspecialties. In general, phase I trials evaluate the safety of new treatments in a small number of patients. If you participate in a phase I trial, an oncologist who specializes in phase I trials will provide care in conjunction with your current oncologist.
Other therapies
Xgeva (denosumab) is a medication used to strengthen bones. It has been shown to reduce the rate of bone injuries (such as fractures, bone pain and new bone lesions) in patients with ADT-resistant prostate cancer who have bone metastases. Xgeva is given as a monthly injection. It can lower calcium and phosphorus levels in your blood, so these must be tested before each dose. It should be taken in combination with a calcium and vitamin D3 supplement.
Xgeva occasionally causes a condition called osteonecrosis (bone damage) of the jaw. If you're currently undergoing or planning dental work (other than a routine cleaning), please discuss this risk with your doctor and dentist.
A group of medicines called bisphosphonates can also be used to prevent the loss of bone density.
Appendix: Summary of FDA-Approved Treatments
The following table shows which treatments are appropriate for treating advanced prostate cancer, depending on whether the cancer is sensitive to androgen deprivation therapy (ADT) and whether distant metastases are present. Please note that these are general guidelines and final decisions are made by the health care provider in consultation with the patient.
| Disease State | ADT Sensitive | ADT Resistant | Usage Notes |
| No Metastases | Lupron® | Lupron® | Injection; ADT 1 |
| Eligard® | Eligard® | Injection; ADT | |
| Zoladex® | Zoladex® | Injection; ADT | |
| Firmagon® | Firmagon® | Injection; ADT | |
| Orgovyx® | Orgovyx® | Oral medication; ADT | |
| Xtandi® | Oral medication; Antiandrogen | ||
| Erleada® | Oral medication; Antiandrogen | ||
| Nubeqa® | Oral medication; Antiandrogen | ||
| Prolia® | Prolia® | Injection; Osteoporosis 2 | |
| Metastases | Lupron® | Lupron® | Injection; ADT 1 |
| Eligard® | Eligard® | Injection; ADT | |
| Zoladex® | Zoladex® | Injection; ADT | |
| Casodex® | Oral medication; Often used briefly when starting Lupron®, Eligard®, or Zoladex® 1 | ||
| Firmagon® | Firmagon® | Injection; ADT | |
| Orgovyx® | Orgovyx® | Oral medication; ADT | |
| Zytiga® | Zytiga® | Oral medication; Antiandrogen (hormone suppressant, generic: abiraterone) | |
| Xtandi® | Xtandi® | Oral medication; Antiandrogen | |
| Erleada® | Oral medication; Antiandrogen | ||
| Taxotere® | Injection; First line chemotherapy (generic:doecetaxel) | ||
| Jevtana® | Injection; Second line chemotherapy (gen:cabazitaxel) | ||
| Paraplatin® | Injection; Can be added to chemotherapy | ||
| Provenge® | Injection; Immunotherapy | ||
| Keytruda® | Injection; Microsatellite instability (MSI) high | ||
| Lynparza® | Injection; DNA damage repair (generic: olaparib) | ||
| Rubraca® | Injection; DNA damage repair | ||
| Xgeva® | Xgeva® | Injection; Osteoporosis and bone metastases in ADT resistant disease 2 | |
| Xofigo® | Injection; Radium infusion for symptomatic bone metastases (Ra-223) | ||
|
1. Trelstar® Vantas® and Viansa® are similar ADT drugs, omitted for brevity 2. Bisphosphonates (e.g., Zometa® and Fosamax®) can also be used for bone health |
|||
This article was written by UCSF medical experts Rohit Bose, MD, PhD, and Arpita Desai, MD, and UCSF patient advocates Bruce Zweig and Stan Rosenfeld. It was last reviewed in May 2022.
This information is for educational purposes only and is not intended to replace the advice of your doctor or other health care provider. We encourage you to discuss any questions or concerns you have with your provider.
















